
1
CHEMISTRY 101
FUNDAMENTAL CHEMISTRY I
BULLETIN INFORMATION
CHEM 101 – Fundamental Chemistry I (4 credit hrs)
Course Description:
A science elective surveying inorganic and solution chemistry. First of a terminal two-semester
sequence.
Note: Three lecture, one recitation, and two laboratory hours per week.
SAMPLE COURSE OVERVIEW
CHEM 101 is an introductory course in chemistry designed to provide a fundamental
understanding of chemistry; its purpose is to prepare students for higher level chemistry classes
by introducing them to basic chemistry concepts and calculations. The material centers on the
basics of matter and its changes and relates this information to medical, engineering and other
fields of work and study. This course consists of a lecture and laboratory portion. The lectures
will include demonstrations, interactive questioning sessions, and problem-solving practice.
Each laboratory will include a homework question session, pre-lab discussion, procedural
explanation, safety requirements and precautions, and a student-led experiment.
ITEMIZED LEARNING OUTCOMES
Upon successful completion of CHEM 101, students will demonstrate…:
1. A good understanding of the scientific method
2. A broad understanding of the fundamental concepts of chemical bonding, reactions,
and practical applications
3. The ability to recognize and understand the impact that chemistry has on every aspect
of their lives
4. A working knowledge of chemistry especially as it relates to the world around them
5. The ability to read popular media and understand the significance of chemistry as it
relates to the world around them
…by specifically being able to:
6. Classify matter and relate its classification to physical and chemical properties.
7. Relate the properties of elements to their structure, location on the periodic table, and
natural state.
8. Measure quantities in the laboratory using appropriate equipment and perform
calculations preserving the precision of those measurements.
9. Identify the bonding characteristics of substances based upon their properties and
elemental makeup.
10. Perform quantitative calculations to predict projected yields of reactions with regard to
products, masses, and energy output or consumption.
2
11. Calculate specific concentration ratios and predict the dependence of reaction
mechanisms (rate and direction) on relative quantities.
12. Identify acids and bases as to their properties and reactions, as well as methods to
determine the concentration of acids and bases.
13. Apply theoretical ideas studied to practical situations in the laboratory.
14. Perform data collection and analysis drawing meaningful conclusions from the data as
part of a cooperative group in the laboratory.
SAMPLE REQUIRED TEXTS/SUGGESTED READINGS/MATERIALS
1. Stoker, S.H. General, Organic, and Biological Chemistry, Sixth Edition, 2010. Houghton
Mifflin. Boston, Mass.
2. Bundy, Robert, Castiglia Lab Manual for Fundamental Chemistry I, Chemistry
101, 2014-2015 Edition.
3. Safety Goggles
4. Scientific Calculator - This must have logarithms and exponential functions.
SAMPLE ASSIGNMENTS AND/OR EXAMS
1. 3 Hour Exams: There will be 3 exams covering lecture topics, reading assignments,
laboratory experiments, and assigned homework. Each exam will be approximately 40 –
50 questions (short answer, multiple choice and/or problem solving.)
2. Final Exam: The final exam is a cumulative exam covering material from the entire
semester.
3. Homework: Homework assignments will consist of practice exercises, examples,
questions, and problems associated with the readings to be covered in the next lecture.
Homework assignments will be graded, and this material will be tested.
4. Lab: Each laboratory will include a homework question session, pre-lab discussion,
procedural explanation, safety requirements and precautions, and a student-led
experiment. For each laboratory, student assignments include pre- and post-lab
questions and a lab report. The lab component will include 14 labs, which consist of lab
reports, exercises, and discussions of research methodology
SAMPLE COURSE OUTLINE WITH TIMELINE OF TOPICS, READINGS/ ASSIGNMENTS,
EXAMS/PROJECTS
DATE CHAPTER TOPIC HOMEWORK
Class 1 1.1–1.4 Course Overview, Intro to
Chemistry
*Memorize elements in pink (p. 13)*
Chapter 1 # 3, 9, 17, 27, 33, 49, 65, 67, 69,
81
Class 2 1.5–1.9
2.1–2.4
Basic Concepts About Matter
Measurement *Memorize metric prefixes in pink (p. 26)*
Chapter 2 # 4, 9, 13, 22, 23, 24, 25, 26, 31,
45, 49, 51, 55, 77, 80, 91, 95, 105
Lab 1 Lab Safety & Lab Orientation
Class 3 2.4–2.8 SF Calculations, Conversions
Class 4 2.8–2.9 More Conversions
Lab 2 Measurement & Physical
Properties
Class 5 3.1–3.5, 3.9 Atomic Structure Chapter 3 # 1, 5, 13, 15, 27, 31, 33, 41, 57,
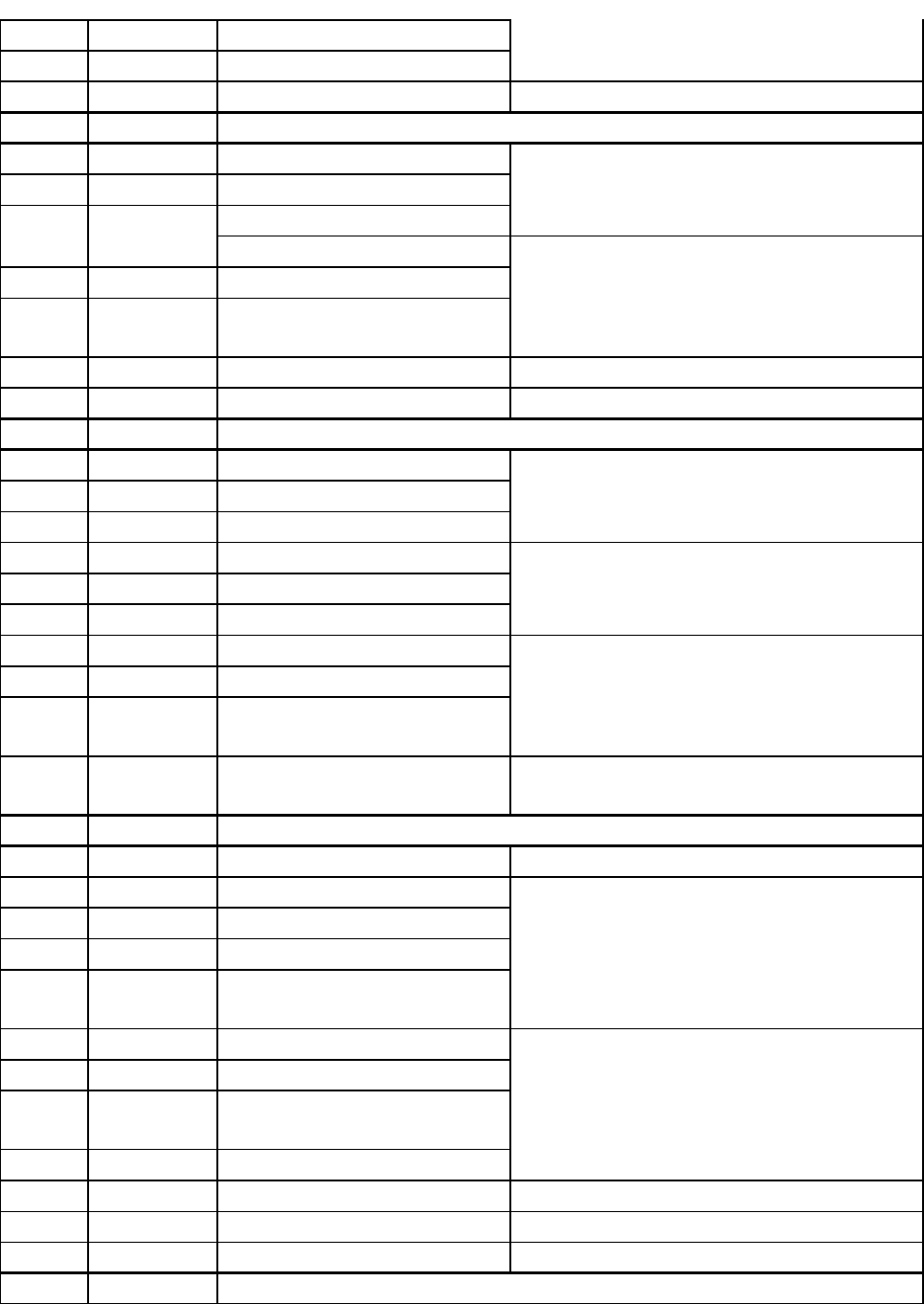
3
59, 71, 73, 75, 81, 83, 99
Class 6 3.6–3.8 Electron Configuration
Lab 3 Elements
Class 7 1–3 Review Study!
Class 8 TEST 1
Lab 4 Ionic & Covalent Compounds Chapter 4 # 1, 2, 3, 5, 11, 13, 23, 27, 39, 53,
57, 61, 79, 83, 87, 95, 107, 113, 115
Class 9 4.1–4.8 Ionic Bonding
Class 10 4.9–4.11
5.2, 5.2, 5.6
Ionic Nomenclature
Covalent Bonding Chapter 5 # 1, 7, 13, 25, 27, 29, 47, 49, 61,
65, 79, 89, 93, 94
Lab 5 Qualitative Analysis
Class 11 5.3, 5.4,
5.8–12
Geometry, Polarity,
Nomenclature
Class 12 4–5 Review Study!
Lab 6 Covalent Molecules Study some more!
Class 13 4–5 TEST 2
Class 14 6.1–6.6 Calculations, Moles, Reactions Chapter 6 # 1, 7a, 9, 13a, 17, 23a, 33, 35,
47, 55, 61a, 75a, 81, 87, 91
Lab 7 Chemical Reactions
Class 15 6.7–6.9 Stoichiometry
Class 16 7.1–7.6 Gases, Gas Laws Chapter 7 # 1, 2, 5, 11, 18, 21, 25, 29, 33,
45, 57, 61, 73, 81, 85, 91, 92
Lab 8 Stoichiometry
Class 17 7.7–7.12 Gas Laws, Phases
Class 18 8.1–8.4, 8.7 Solution Formation Chapter 8 # 3, 5, 7, 9, 11, 15, 17, 19, 21, 23,
25, 33a, 40, 53b, 55a, 66a, 77, 83, 95, 105
Lab 9 Gases & Gas Laws
Class 19 8.5–8.6,
8.8–8.9
Concentration, Colligative
Properties
Lab 11 Solutions
Review 6-8 Study!
Class 20 6-8 TEST 3
Lab 10 Determination of R
Class 21 9.1–9.3 Redox Reactions *Memorize ox. number rules (p. 242-3)*
Chapter 9 # 3, 7, 8, 11, 13, 14, 18, 23, 25,
32, 46, 49, 61, 64, 70, 83, 88
Class 22 9.4–9.7 Reaction Energetics, Equilibria
Lab 12 LeChâtelier’s Principle
Class 23 9.8–9.9 Equilibrium Constant,
LeChâtelier
Class 24 10.1–10.6 Acids, Bases, Salts *Memorize strong acids & strong bases
(p. 277-8)*
Chapter 10 # 1, 2, 3, 4, 6, 7, 12, 15, 20, 25,
30, 33, 35, 42, 44, 45, 51, 56, 61, 63, 73, 77,
97, 109, 113, 125, 129, 147
Lab 13 Acids & Bases
Class 25 10.7–10.9,
10.15, 10.16
Neutralization,Titration
Class 26 10.10–10.14 Buffers
Class 27 12, 13 Introduction to Hydrocarbons Study…
Lab 14 Final Exam Review Keep studying…
Class 28 1–10 Final Exam Review Study some more…
1–10 Final Exam according to University exam schedule

4
